What You Should Know:
– Better Medicine, an AI-powered diagnostic startup for radiology, has secured nearly €6M ($6.89M) in total funding. The company recently raised €1M ($1.1M) in a pre-seed funding round led by Soulmates Ventures, with additional participation from Specialist VC, UT Ventures, and several angel investors.
– The funding follows a €2.5M ($2.9M) grant from the European Innovation Council (EIC) and other grants, bringing its total funding to €2.25M ($2.6M) in private funding and €3.7 million in grants. This capital will support the company’s commercial rollout in Europe, expansion of its product portfolio, and preparation for FDA clearance.
Addressing the Radiology Crisis with Clinically Validated AI
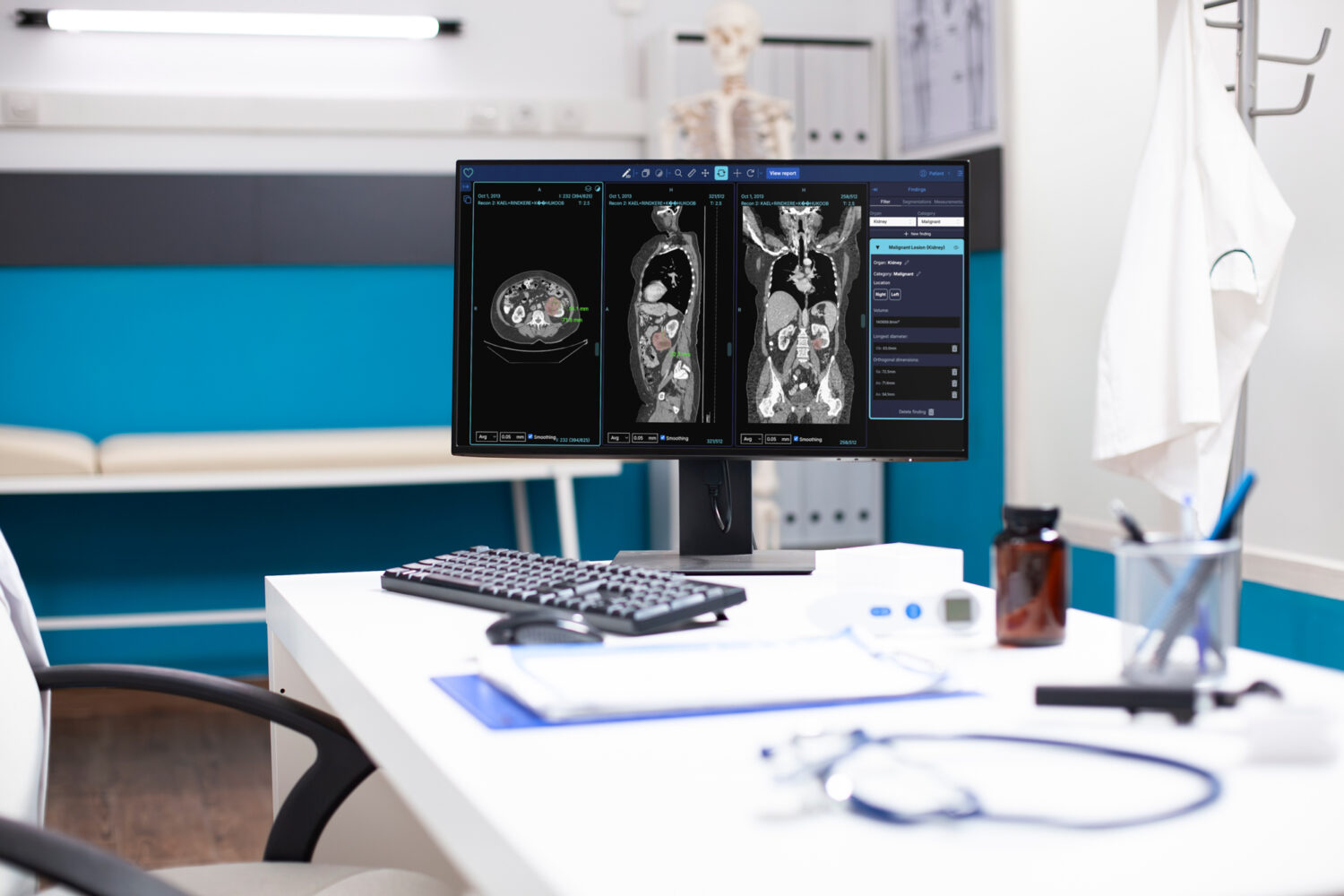
Radiology departments worldwide face immense pressure due to a growing volume of scans and a shortage of qualified professionals. In the UK, a recent survey found that 50% of radiology job postings go unfilled. Better Medicine’s platform addresses this by deploying clinically validated AI models that assist radiologists with the detection, measurement, and reporting of oncological findings in CT scans. The technology allows for automated lesion detection and measurement and integrates seamlessly into standard radiology infrastructure.
The company’s flagship product, BMVision Kidney, recently received CE certification as a Class IIa medical device under EU regulations, making it the first CE-certified, AI-based kidney cancer detection tool to comply with the regulation. Clinical evaluations showed that radiologists using BMVision reported up to 52% time savings and a 99.2% detection rate.
Global Expansion and Future Outlook
The company, founded in 2020 and headquartered in Tartu, Estonia, has a global team across six countries and has secured clinical collaborations with partners including Leeds Teaching Hospitals NHS Trust and Tartu University Hospital.
The new funding will be used to support Better Medicine’s commercial rollout across multiple European countries and expand its product portfolio to cover additional organs and metastatic sites. The company is also preparing for FDA clearance and plans to launch clinical pilots in the U.S. that are tailored to meet FDA study requirements.
“Imagine a second set of eyes, but multiplied by 1000, always alert and never tired,” said Priit Salumaa, Founder and CEO of Better Medicine. He emphasizes that the technology is designed to make early cancer detection easier and free doctors from repetitive tasks to focus on what requires their clinical judgment.


